From production platforms to oil strings, corrosion begins the day the steel is cast, meaning that a protection strategy must be implemented from the outset. Offshore structures immersed in seawater can be guarded against corrosion using cathodic protection. Structures such as oil platforms, which exist both in and out of the water due to tidal changes, benefit the most from this form of protection, since exposure to a combination of saltwater and air accelerates degradation due to corrosion. Different strategies exist for cathodic protection, including using an impressed external electrical current or sacrificial anodes; however, due to their simplicity, sacrificial anodes are often the preferred method.
Cathodic protection
Cathodic protection using sacrificial anodes follows a simple strategy: a steel structure such as an oil platform is electrically connected to a less noble metal, for example aluminium. When immersed in seawater, which acts as an electrolyte, the sacrificial anode becomes anodically polarised and the steel structure cathodically polarised. The anodes are dissolved through anodic dissolution of the metal, while oxygen reduction takes place on the surface of the steel structure. The diagram on the left (page 13) shows a typical oil platform geometry, where 40 cylindrical sacrificial anodes have been placed relatively close around the oil platform. The polarisation of the anodes and the oxygen reduction reactions occurring on the surface of the steel structure are relevant for corrosion protection. Corrosion protection is achieved at the point where the cathodic current is equal in magnitude (but opposite in sign) to the anodic current. The current density for oxygen reduction is limited by the oxygen supply. The result is a near-constant, transport-limited current value over a few hundreds of millivolts in potential at the surface of the steel structure. As long as the oxygen reduction taking place on the surface of the steel remains within the corrosion-protected range, the cathodic-limiting current causes the sacrificial anode to be corroded and provides a degree of protection for the oil platform structure.
Corrosion protection simulation
In order to fully protect the platform, design engineers need to ensure that the different parts of the structure are well within the corrosion-protected range. In other words, the voltage supplied by the anodes needs to be sufficient to drive oxygen reduction at the steel and balance the current. The first step when designing the protection system is to investigate the potential of the steel structure assuming a constant cathodic current (oxygen reduction). This ensures that the anodes will be able to supply the necessary potential to maintain the given current. The potential has to be well within the required range in which oxygen reduction protects the structure and hydrogen evolution is avoided.
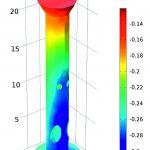
Multiphysics simulation can be used to predict the electrolyte potential on the surface of the anodes and the steel structure (cathode). The surface plot in the graphics shows how the potential varies by several hundred millivolts depending on the position of the anodes. The lower the electrolyte potential at the interface, the more positive the potential difference between the oil platform and the seawater – an expected result since the current in the electrolyte flows from the anodes to the cathode. In the close-up of one of the structure’s legs it can be seen that the inside bottom part has the lowest potential, indicating that this section of the leg will be the part of the structure most susceptible to corrosion.
Understanding corrosion processes
Comsol Multiphysics simulation software can be used very effectively to gain insights into the current and voltage distribution of electrochemical systems. In this model, the electrolyte potential was first obtained using an approximation that assumed constant current density in the cathodic structure. The model demonstrates that the lower the electrolyte potential, the less protected the structure will be from corrosion. By understanding this relationship, the model is able to give a good overview of the less protected and therefore critical parts of the oil platform. The model can be easily expanded to take into account additional effects such as overpotential or concentration gradients, which can be utilised to develop a more detailed understanding of the corrosion processes.
Online search: cppPC117comsol
Facts & Figures : Software tool for Simulation
Version 5.3 of Comsol Multiphysics makes the boundary element method (BEM) available for modelling electrostatics and corrosion effects. The boundary element method enables users to simulate models with infinite domains and voids as well as to quickly set up simulations that combine wires, beams, surfaces and solids in the same model. The typical uses for this functionality include modelling electrical cathodic protection, cables or capacitive sensors. In addition, users handling large CFD models will benefit from the new algebraic multigrid (AMG) solver implemented in Version 5.3. The AMG solver is now the default option for many fluid flow and transport phenomena interfaces. Users modelling turbulent flows can profit from more robust computations with the automatic treatment of walls. This feature blends a high-fidelity low-Reynolds formulation with wall functions.