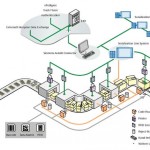
About 10 % of all drugs sold globally are fake – equivalent to a massive 200 billion USD annually. The industry first began to tackle the problem of counterfeit prod-ucts some time ago, with serialisation being its most important weapon. With stricter legislation meanwhile becoming effective in a growing number of countries and multinational companies looking for universal ways to code their products around the world, item level serialisation is now ready for the next step.
The author: Hans Bijl Sr. Manager Business Development Life Science, Siemens
The preferred way of dealing with the increasing number of counterfeit medicines and meeting new regulations is to secure the supply chain of individual drug packages with the help of technology. As a first step, item serialisation (individual numbering at item level with a unique and tamper-proof ID) has evolved over the last few years as the technology of choice. There is not a single large drug company today that does not take serialisation seriously – and act accordingly. Tracking and tracing task forces and project teams have been set up, pilot lines built and – in case the company happens to serve regions that are already affected (e. g. France with CIP13 coding or Turkey with coding for both serialisation and aggregation) – actual production lines equipped to manufacture single item-numbered products.
Unfortunately, despite the global nature of modern drug supply chains, the legislation in force is highly fragmented and local. In many cases new legislation is merely announced and there is one delay after another before it is actually implemented. One of the most obvious examples here is Brazil, where national legislator Anvisa proclaimed local regulations (based on tamper-proof labels with a 2D barcode) back in 2009, to become effective in January 2011. In the meantime, however, a new Brazilian government has taken office, resulting in the postponement of the promised regulations earlier this year and it is now anyone’s guess when they will actually become compulsory.
Both the EU and the US would like to establish the universal GS1 standard for item level numbering in combination with Data Matrix (2D barcode) coding, with effective dates as late as 2014 and 2015. And although it will represent a major step forward in terms of standardisation, the legislation proposed in the EU is currently no more than a directive which does not tell the industry exactly what it needs to be prepared for – or when. The most recent development in this connection is the German SecurPharm initiative. Against the background of EU announcements, a formal group comprised of the German pharmaceutical industry, regulators, distributors and pharmacies has set up a dedicated organisation and launched a campaign to formulate a comprehensive, end-to-end verification solution. The initiative partly builds on the experience gathered in 2009 with the EFPIA pilot project, where suppliers (including Siemens) constructed a similar, though smaller, first pilot in the Greater Stockholm area. If the German project is successful, it will probably also set the standard for other European countries. The level of serialisation is likewise variable. Whereas some regions only require item level serialisation with an individual number printed on each package, other countries go one step further. They insist on aggregation, where hierarchical information that is generated whenever items are packed into bundles or cartons and on pallets is linked together and stored accordingly. This clearly affects the technological requirements relating on the one hand to data handling and on the other to the interfacing between the individual steps in the packaging process.
Despite the above-mentioned variability and uncertainties, the industry acknowledges that serialisation is “here to stay”. The challenge is to establish a serialisation infrastructure that goes beyond the mere numbering of an individual package. Global, comprehensive and secure handling of packaging data should not only comply with various serialisation requirements but also increase supply chain efficiency. There are three key aspects that need to be considered.
1 – Technical Solution
Standardisation can provide the answer here. However, since this would appear to conflict with the desired variability, what the industry really needs is a solution that enables it to switch from a “wait-and-see” attitude to “wait and be prepared”. Technical concepts should preferably be built around standard components and functions while offering the necessary adaptability to meet requirements that are as yet unknown. Flexibility and modularity are vital. Highly standardised, tested and modular components that support fast and versatile implementation are a must for any company endeavouring to implement a serialisation infrastructure in its packaging processes in a harmonised, cost-effective and efficient way yet still be in a position to respond to upcoming or changing regulatory requirements.
2 – Organisation
In addition to the right technical solution there is also a growing awareness in the industry that a truly global rollout is impossible without a supplier that is capable of handling such an implementation. Building a pilot line is one thing – managing a rollout of sixty lines in four different regions, with three different levels of aggregation and a time frame of just eighteen months, calls for an altogether different approach.
The side-effects of serialisation should not be underestimated. The fact that in most cases introducing a serialisation solution will affect existing packaging lines and workflows means that production managers often adopt a critical attitude when examining the planned changes and the likely repercussions for the overall equipment effectiveness (OEE) of their packaging lines – not to mention the negative impact on current production. Choosing a supplier that takes such misgivings seriously and is used to working in a validated, critical environment like a pharmaceutical packaging plant will help reduce the potential risks and, in the end, lower the cost of implementing the serialisation solution. Finally, a glance at the worldwide implementation calendar (presently showing a peak in effective dates for regulations between late 2013 and the end of 2015) confirms that the global availability of qualified resources is another area for concern.
In the light of all these restrictions and challenges, the industry is on the lookout for partners rather than just suppliers. What is wanted are organisations that are willing to assume responsibility for the successful implementation of their serialisation platforms – regardless of the location, size, level, technology and number of subcontractors.
3 – Cost effectiveness
At the moment, the industry tends to see item level serialisation as yet another obligatory regulatory hurdle – one that will simply add to the enormous amount of money already spent annually on keeping pace with a variety of regulations around the globe. This is especially true as long as the downstream validation technology has not been installed (at the point of sale or point of care). Manufacturers and regulators will only really be in a position to tackle the problems associated with counterfeit medicines with initiatives that cover the entire supply chain right the way down to the sale or care point.
There are other ways of looking at this, however. Item level serialisation can contribute to overall supply chain optimisation – as one way to deal with the business related issues mentioned earlier. Interesting replies gathered du-ring a 2005 study revealed that acceptance of serialisation as an anti-counterfeit measure would increase if, in addition to compliance with regulations, the improvements would also save money by making the supply chain more transparent.
In order to exploit these opportunities and make serialisation a chance to be seized rather than a mandatory must-do, all the above conditions need to be met: a standardised solution using standardised components, yet configurable with the maximum possible flexibility and modularity and offered in combination with a service level that enables a true global rollout and lifecycle support. Only by forging partnerships with suppliers like Siemens that fulfil the above requirements will the industry be able to cope with the wave of serialisation that is already looming on the horizon – and leverage the potential for supply chain optimisation that lies underneath. Ever since pharmaceutical regulations first pointed towards item level serialisation as a suitable measure, Siemens has been applying its expertise and products in this field to develop a suitable answer to the issues of the twenty-first century. The outcome is a comprehensive solution for both serialisation and aggregation.
cpp-net.com/0312457
Share: