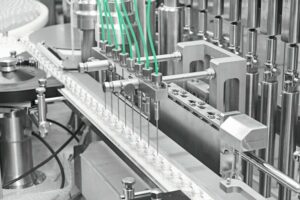
Existing production lines often require modification for new and innovative products. In cooperation with special machine manufacturer Bausch+Ströbel the NSM 9060 was developed in 2008 for GP Grenzach to label syringes, insert the plunger rods and assemble the needle guards. As market demand remains high and the machine design has proved successful, GP recently decided to invest in a second machine of the same type.
The author: Tanja Bullinger Head of Public Relations Department, Bausch +Ströbel
GP formerly belonged to Hoffmann-La Roche AG but has been an independent subsidiary of Bayer Health Care since 2005. High-quality medicines, cosmetics, dietary supplements and medical products are produced and packed there daily for national and international distribution. Bayer products are likewise processed at this facility: one of the most famous is the Bepanthen product line. Moreover, several big-name pharmaceutical companies have their products manufactured, packed and distributed by GP.
Processing single-use syringes is a major mainstay of the company; several million syringes leave the factory every year filled with chemical or biotechnological products and the trend is rising. Seventy percent of these single-use syringes are equipped with a needle guard, which is a safety device to protect the user from needlestick injuries after administering the injection.
“When one of our customers was seeking to have his syringes equipped with a needle guard in 2007, this meant that GP had to convert the complete production line promptly to ensure that there was no interruption in the market supply. We managed to adapt the production process to meet this requirement in record time and we were thus able to guarantee a constant flow”, recalls Burkhard Hunold, Head of Project Management at GP, not without pride. “Due to the steadily growing demand for single-use syringes with needle guards, our capacity urgently needed to be increased. We were also keen to improve the machine design for the new packaging line even further and bring it into line with current GMP standards”, explains Markus Korneli, who is responsible for Packaging & Services at GP Grenzach.
“Bausch+Ströbel was the only supplier back then to provide labelling, plunger rod insertion and the application of the new needle guards not as several machines but integrated in a single, compact, high-capacity machine”, Hunold adds. “The production area at GP is limited and therefore precious.” As cooperation had been positive in the past, GP wanted the machines for the new production line to be designed by B+S engineers again, taking various special requirements into account. Although GP is not generally in favour of installing prototypes, the company trusted Bausch+Ströbel’s long experience in the construction of customised machines.
Integration of a new step
It was basically a matter of integrating an additional working step into the type SME 2018 syringe assembly and labelling machine that GP already had on order. “After labelling the syringe and applying the plunger rod, the syringe had to be inserted into the new needle guard”, observes Martin Müller, the man in charge of the project at Bausch+Ströbel. And so B+S developed the NSM 9060 machine type and implemented the requirements specified by GP. It was the details, in particular, that testified to the manufacturer’s technical know-how and extensive experience: both the plunger rods and the needle guards feature a special geometry that is very important for processing. The machine has two vision systems: one to check the variable data printed on the label and the other to verify from two angles that the needle guards have been assembled correctly and are intact. The check stations familiar from other B+S labelling machines are also integrated. More cameras can be installed as an option, for example to inspect the syringes for damage. The NSM 9060 can additionally apply a label to the needle guards themselves as a final step. “We haven’t used that option yet”, says Korneli.
The FAT confirmed that the right manufacturer had been chosen: the new model passed all performance tests at the first attempt. Hunold recollects that no modifications were neces-sary following the final acceptance.
More than simply speed
The NSM 9060 is capable of processing up to 24 000 containers per hour. However, there is more to it than simply speed. It is obviously essential for the machine to run trouble-free and offer the lowest possible reject rate. Korneli illustrates this with the help of an expensive, high-grade product requiring refrigeration: “This product is only allowed to be outside the cold store for a strictly limited period of time. If the machine is down for longer, it must be completely cleared and the product returned to the cool area to be on the safe side. The equipment also had to fit in with the production process overall. Downstream of the NSM 9060 is a blister packaging machine. Since it has a lower output than the NSM 9060, an SPE 8090 buffer was interposed by Bausch+Ströbel; it works according to the first-in/first-out principle and compensates for any fluctuations in the process, so that the blister machine runs continuously.
In the meantime the NSM 9060 operates round the clock in Grenzach in five shifts – with virtually no problems of any kind. The operators are very pleased with the machine and praise its clear layout. Thanks to the gentle transport system using suction wheels, damaged containers are kept to a minimum. GP is highly satisfied with the quick and reliable service provided by the manufacturer if ever a malfunction occurs or a setting needs to be changed.
“Of course there’s always something that could be improved”, Korneli comments. The experience gained with the first GP machine has been incorporated into the planning of the second. Both Burkhard Hunold and Markus Korneli emphasise that the basic design was fine and that only a few minor details are up for discussion. Another crucial aspect for GP was versatility. The pharmaceutical market is forever changing and contract manufacturers are regularly confronted with new customer specifications. If the needle guard being processed is replaced with an alternative safety device, it can still be handled by the NSM 9060 without any major conversion work. All popular needle guards currently available in the market (e.g. from BD, Safety Syringes Inc. or Plastef Investissements SA) are compatible with the NSM 9060.
cpp-net.com/0213456
Share: