L.B. Bohle Maschinen + Verfahren from Ennigerloh, Germany, has developed solutions for the continuous production in the pharmaceutical industry. Bohle started off with a continuous granulator and has now come up with a twin-screw extruder with a vacuum contact dryer. The extruder has been tested by scientists at Bonn University. An interview with Prof. Dr. Klaus-Jürgen Steffens on the extruder and tests follows below.
cpp: In pharmaceutical solid dose manufacturing, switching from batch to continuous production offers a lot of advantages. Professor Steffens, you have already carried out a number of tests with the BCG (Bohle Conti Granulator). Could you explain the system’s basic operating principle to us?
Steffens: The core of the system is a newly designed GMP-compliant twin-screw extruder, which is supplied by a continuous mass-controlled powder dosing unit and a continuous liquid dosing unit. The finished granulate drops directly into the continuous rotating vacuum contact dryer, without requiring transport. The dryer operates with an innovative blending and dispersing unit which reliably prevents the wet granules from caking to surfaces. The final granule size is then adjusted using the well-known Bohle BTS turbo sieve.
cpp: Which tests have you carried out with the system so far?
Steffens: We carried out tests with substances that are difficult to granulate, such as Paracetamol and Ibuprofen at a high dosage of 75 % active concentration. We compared the results with high-shear granulation and tested various binding agents as well as the impact of different screw configurations. Our targets were always the properties of the tablets produced from the granules, such as strength (tensile strength is independent of tablet size), disintegration and substance release.
cpp: Can you tell us about the initial results?
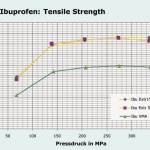
Steffens: Out of the many tests, a few results stand out. Regarding the strength of Ibuprofen tablets which were granulated with PVP as the binding agent, two throughput rates in the extruder were compared to high-shear granulation (VMA). Our measurements confirm the clear superiority of extrusion thanks to the better binding agent distribution. The same applies to Paracetamol, where the higher throughput in the extruder led to even higher strength values.
We carried out a further test on the tablet strength of a Paracetamol formulation granulated with HPMC 4000 as the binding agent instead of PVP. Surprisingly, virtually the same strengths were recorded as with PVP, although with extrusion the binding agent is normally a dry powder mixture which must first be dissolved in the extruder. We actually expected major problems with the tablet strength, as HPMC 4000 has a much lower dissolution rate in water than PVP. Specimens A to F were granulated with different screw configurations and different amounts of liquid. Only specimen E showed a significant tablet capping tendency. This specimen was produced with the least amount of liquid. De-spite having practically the same strength values (in the region of 2.5 MPa), the tablets from the other specimens revealed varying disintegration times ranging from 133 s (specimen F) to 390 s (specimen A). Scanning electron microscope images showed the typical structure of the granules, which is no different from that of other granulating processes.
cpp: What are the benefits of the Bohle system in your opinion?
Steffens: In contrast to other extruders the BCG extruder is characterised by the typical Bohle GMP design. The monoblock design has no gaps or connections, making the equipment easy to install and clean. The “real” torque measurement – separately for each screw – is particularly noteworthy, allowing outstanding control of the process. This is another step forward in implementing process analytical technology. Last but not least, the new continuous rotating vacuum dryer of the BCG system features significant advantages compared to other systems.
cpp: How would you rate the other solutions available on the market? Which features do they have in common with the Bohle technology and how do they differ?
Steffens: So far, a number of tests have been carried out for continuous wet granulation, such as the continuous fluid bed system or the semi-continuous process utilising very small, pulsed batches. They have not taken hold of the market for wet granules because of their low shearing force and complicated, failure-prone transport systems.
Extrusion, on the other hand, is a promising solution. The shearing forces can be much better tuned to the product properties during granulation than with any other process.
cpp: What are the future trends in pharmaceutical production?
Steffens: The pharmaceutical market will be dominated by dualism. On the one hand, we will have innovative and expensive pharmaceuticals that will be produced in relatively small batches and for which the production costs will be less important. At the same time, we have observed global cost pressure and a high concentration of manufacturers in the constantly growing market for generic drugs. This will result in increased market shares for cost-cutting, continuous production systems. It is therefore vital that the control and machine technology of these systems is state-of-the-art to ensure elegant and enhanced, fail-ure-free production.
Online-Info: www.cpp-net.com/0311421
Share: