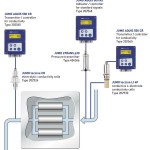
Pharmaceutical water is needed for a variety of different applications – whether in tablet production, for making ointments or when cleaning systems. Pure water is the primary ancillary medium. Without pharmaceutical water, it would not be possible to produce most active ingredients, and its quality is a precondition of consistently high product quality. Different procedures are used to manufacture it and nowadays, reverse osmosis is the most popular method.
The authors: Dr. Öznur Brandt Product Manager, Business Unit Analytical Measurement, Jumo Christina Hoffmann Market Segment Manager Pharma & Food, Jumo
Osmosis is based on the principle of equalising the concentration of two liquids of initially different concentrations with the aid of a semi-permeable membrane. The basis for this is the osmotic pressure of the liquids. This principle is exploited in reverse osmosis. A correspondingly higher pressure is used to push the water through a membrane against the natural osmotic flow and the ions and minerals present in the water are left behind. Regular process and quality control are necessary for quality assurance. The pressure and conductivity are monitored and recorded during the process.
Pressure measurement
Pressure monitoring is imperative for the efficiency and success of a reverse osmosis system. Pressure is needed to push the water through the membrane against the osmotic pressure. Its strength depends on the composition of the water and the size of the system. The osmotic pressure of drinking water is approximately 2 bar, which means pressure in the 4 to 30 bar range is required for reverse osmosis.
The Jumo dtrans p30 is ideal for the measurement required here. The pressure transmitter is used for applications that demand a combination of reliability and long-term stability, which is also the case with reverse osmosis. The measuring system is fully welded and does not need seals, thus ensuring maximum process safety. The crucial element is a piezoresistive measuring cell which, thanks to its high overload resistance and excellent long-term stability, is safe, reliable and temperature-resistant. With a broad spectrum of measurement ranges and electrical outputs as well as numerous hygienic process connections, the number of different models is so vast that it is possible to find an individual solution for every application.
To comply with the quality requirements of pharmaceutical water production, all parts of the Jumo dtrans p30 that come into contact with the medium are made from stainless steel 1.4435 (316L) and can be supplied in electropolished quality (. 0.8 µm).
Conductivity measurement
Pharmaceutical water, also known as ultra-pure water, is water in its purest form, that is without any impurities whatsoever. This water must have a conductivity value below 1 µS/cm (at 25 °C). The quality of ultra-pure water (purified water, water for injection, etc.) is described in standards and recommendations such as those of ASTM International (formerly the American Society for Testing and Materials) or in the European Pharmacopeia (Ph. Eur.), the United States Pharmacopeia (USP) and DIN or ISO. All the standards mentioned here demand appropriate quality controls.
The stainless steel version of the high-quality Jumo tecline CR conductivity cell is the ideal solution for measuring conductivity in ultra-pure water, which is the safest and most reliable quality control method of all. Two models are available, with cell constants K = 0.01 and K = 0.1. The K = 0.01 version covers a measurement range from 0.05 to 10 µS/cm. It is therefore well-suited for use in pure and ultra-pure water applications.
All parts of the instrument that come into contact with the medium are made from electropolished-quality stainless steel 1.4435 (316L). Inspection certificates in compliance with EN 10204 3.1 can be provided together with roughness certificates (. 0.8 µm as per Ph. Eur. and ASTM International). The seals and plastics that are used meet the requirements of the FDA and are physiologically safe. The cell constant can be measured and confirmed by an ASTM test certificate. This is particularly important for pharmaceutical water systems.
As electrolytic conductivity is strongly temperature-dependent, temperature has to be included in the evaluation. A Pt1000 sensor is integrated into the conductivity cells for this purpose. During the measuring process, this sensor detects the temperature of the medium, which is then compensated accordingly in the transmitter.
The Jumo Aquis 500 CR is a particularly suitable transmitter and controller for pharmaceutical water applications. It is used for conductive measurements as well as for controlling electrolytic conductivity and specific resistance. The instrument has the following capabilities for conductivity measurement in ultra-pure water:
- Numeric (precise) input of cell constants: When the measuring point is put into service, all that must be done is to programme the exact cell constant into the transmitter; the measuring cell is then ready to use.
- Temperature compensation to ASTM D 1125-95: The standardised test and analysis procedures of the ASTM International organisation also define methods for determining the electrolytic conductivity of water and ultra-pure water: D (designation) 1125-95. This publication additionally specifies the dependence of the conductivity measurement on temperature as well as on different types of impurity. Formulae for the latter are included in the Jumo Aquis 500 CR operating software.
- Limit monitoring to USP (water conductivity ,645.): With the Jumo Aquis 500 CR transmitter/controller, it is possible to monitor the quality of ultra-pure water online in accordance with the USP Stage 1 specification. The USP includes a table that specifies a limit value for conductivity as a function of temperature. Providing the conductivity stays below this value, the ultra-pure water meets USP requirements.
The Jumo Aquis 500 CR has a plain text user interface. Parameters shown in plain text simplify configuring and help the operator to program the instrument correctly. The Jumo Aquis 500 CR with calibration certificate and the Jumo tecline CR complete with pharmaceutical package is a successful combination in line with current requirements. The pharmaceutical package comprises:
- Materials test certificate to EN 10204 3.1
- Roughness test certificate to EN ISO 4287
- ASTM test certificate for precisely gauged cell constants
Recording
The Jumo Logoscreen es with FDA-compliant data recording is the right product for documenting the pharmaceutical water production process securely. In combination with its PC software components, this paperless recorder forms a closed system for electronically entering, saving and archiving process data in conformity with FDA 21 CFR Part 11.
Its appearance is dominated by a 5.7” colour display, on which the measurement data appears in various modes (numeric, chart or bar graph). The integrated Security Manager prevents unauthorised persons from operating the instrument while the inclusion of the Audit Trial Manager ensures that all actions are documented without interruption. The measurement data is saved electronically and made available both for on-site evaluation and for analysis on a PC.
Hall 1, Booth 137
Online-Info: www.cpp-net.com/0311403
Share: