Many products, especially vaccines and therapeutical substances, are manufactured with the aid of biotechnological methods. This means that increased attention must be paid to the biologically contaminated waste water generated by these processes. This article presents the current state of the decontamination process and describes the advantages offered by systems in which all stages, from the production of ultra-pure water to the disposal of the waste water, are supplied by a single manufacturer.
Helmut Sommer
Depending on the biological safety class (BL 1, BL 2 or BL 3), all waste water from a biotechnological production system must be treated – in general, this means sterilised – such that no microorganisms can enter the factory’s waste-water treatment plant and that there is absolutely no potential contamination hazard for the environment. This decontamination of the waste water is generally carried out with a batch-mode or continuous sterilisation system. The waste water frequently contains a mixture of fermentation residues, as well as microorganisms and the water used for flushing and CIP. Its composition may vary widely and it may also contain solid particles. The treatment systems must therefore be very robust and designed to be extremely safe. The treatment of the biological waste water is thus a key function of a biotechnological production system.
The main properties required of a decontamination system are:
- Absolutely sterile waste water at all times
- A validated and documented process
- Round-the-clock operation must be pos-sible
- Short batch-processing times
- Quiet operation
- Minimal running costs
- Almost maintenance-free operation
Both batch and continuous systems meet these requirements equally well. Furthermore, batch systems also permit the unambiguous documentation of each batch. The sterile state of each treated batch of waste water can be monitored and documented with the aid of the process controller and by taking samples. In order to permit continual operation and to ensure a high throughput, batch systems can also be constructed as dual systems which run in parallel: while one system is sterilising a batch, the other system is cooling down ready for the next batch.
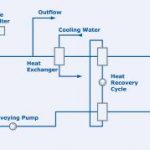
Continuous systems, on the other hand, offer a major advantage in their running costs, since heat can be recovered from the cooling stage and returned to the heating stage. However, since these systems do not permit unambiguous identification of the waste water being treated, they are in general used only for weakly contaminated waste water.
User-oriented from A to Z for trouble-free operation
In order to meet the differing user requirements, decontamination systems should be based on a modular design and each should be planned individually. Basically, just as everywhere in the sensitive areas of pharmaceuticals and biotechnology, the principle is: the fewer interfaces needed for the planning and construction of such systems, the more safely will the systems operate. Integrated systems which combine everything from the supply of ultra-pure water and steam to the treatment of the waste water, guarantee the greatest pos-sible safety. Such a system is a complete pharmaceutical production line from a single source, delivered as a turnkey system. This, in turn, means: fast project execution with the minimum possible number of interfaces, integrated controllers based on PLC systems, which permit maintenance-free and fully automatic operation, and standardised and complete documentation of the entire system, which is manufactured in accordance with GMP. Versions which comply with the documentation steps laid down in 21 CFR Part 11 can also be integrated without problems.
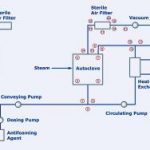
Technical construction of the batch process
In the batch process, the waste water is collected in a buffer tank. When the water there reaches a certain level, it is pumped into the sterilisation autoclave. Here, saturated steam is used to heat the waste water to more than 121.1 °C, thus sterilising it. The design and placement of the steam inlet is critical for the entire process execution and for the noise levels it generates. In order to reduce the noise level and to ensure uniform heating of the complete system, the autoclave should be equipped with a vacuum system where the chamber of the autoclave is evacuated by a vacuum pump before the sterilisation process is started. This ensures, during sterilisation, that the desired temperature is always reached on all surfaces of the autoclave and also in the exhaust-air channels, so that any microorganisms sticking to their walls are also destroyed. This ensures correct sterilisation, where the temperature normally lies between 125 and 135 °C. The process is successfully completed if this temperature is maintained everywhere within the system for at least 30 minutes. All sterilisation parameters can be configured as desired; any changes are documented accordingly and recorded in the process.
A further critical point in such systems is the above-mentioned treatment of the exhaust air leaving the chamber of the autoclave. This air may have an offensive smell and may also be contaminated with microorganisms. In modern systems, this exhaust air is therefore treated in two steps. The microorganisms are first removed by means of sterile filtration and a deodorisation unit removes any unpleasant smells. The sterile filter and the exhaust-air channels are also heated during each sterilisation cycle in order to decontaminate them. The resulting condensate is always returned to the buffer tank, this ensuring that no non-sterile substances can escape from the system. After completion of the decontamination process, the sterile waste water is cooled by means of a plate heat exchanger. When its temperature has dropped to 40 °C, the sterile contents of the autoclave are automatically discharged into the external waste-water system, providing all process parameters were correct. The autoclave is now ready to automatically start and document processing of the next batch.
Since some types of waste water tend to foam when heated with steam, a dosing system for an antifoaming agent is installed in the inlet pipe to the feed pump in such applications. The heating and cooling times must be further optimised in such systems in order to achieve the optimal energy consumption and the maximum throughput.
Liproline system follows turnkey concept
According to the importance of the turnkey concept, ultra-purewater specialist Christ offers with Liproline a decontamination system plus mixing tanks, preparation lines as well as CIP/SIP systems. Liproline is available in versions designed for fully automatic batch or continuous operation. The inactivated waste water can then be discharged into the normal waste-water network of the factory. An exhaust-air deodorisation unit is available as an option in order to prevent unpleasant smells. The Liproline systems for the treatment of pharmaceutical waste water remove the water from the solutions to be treated and thus greatly reduce the amount of materials which has to be disposed of. In addition, the special evaporator technology inactivates certain active substances. Depending on the application, this can also be achieved by low-cost UV oxidisation of the substances with the aid of the UV units of the Liproline range.
Trouble-free commissioning saves time and money
Since ultra-pure water and decontamination systems are subject to the regulations with respect to biological safety, comprehensive installation inspections and functional tests must be carried out. These include checking for compliance with the regulations for pressure vessels, the inspection of welded seams (including the production of test welds), endoscopic examinations and, in some cases, X-ray examinations.
Just as in all other pharmaceutical systems, the system test must be preceded by the installation qualification, in which all individual items, extending from the material certificates to the loop checks and the alarms generated by the PLC are tested. During the FAT (Factory Acceptance Test), thermal mapping is executed at specifically defined positions of the system in order to validate the entire sterilisation process. A further element of the tests is a forced interruption of the process in order to check that the sterilisation process starts again correctly after the interruption. A test of the entire system at full load must also be carried out at the manufacturer’s factory, since this is the only way of reliably testing all later effects of the system. Just as for any sterile production system, the overall qualification of the waste-water treatment system is absolutely necessary in order to meet the requirements of the official authorities. In most cases, the many qualification documents needed for IQ and OQ are provided by system manufacturers like Christ; these are inspected, in cooperation with the subsequent user, as part of the FAT. In the subsequent on-site functional tests, the new system is connected to all supply systems of the manufacturing location and then tested and documented under real operating conditions.
Summary
The increasing quantities and qualities of biopharmaceutical products make higher standards necessary, and this is also true for the manufacture of the systems used in the final stages of processing plants. Fully automatic, qualified sterilisation systems operating in batch or continuous mode ensure reliable operation and must be adapted to meet the user’s requirements for a given application. They should therefore be based on a modular design, like the Christ-Liproline devices, which ideally should also encompass the production and distribution of ultra-pure water. They must also comply with the quality requirements for a production system, since safety is of prime importance in such applications.
Hall 4.1, Booth J8
cpp 457
More about Liproline
All about Biotechnology
Share: