Whether in blood pressure tablets or sleeping pills – highly active substances are staple ingredients in pharmaceutical production. Although several approaches to the design of process plants for solid forms handling are available today, preventing participant contamination in each of the phases remains the key planning goal.
Jean-François Gass, Jörg Wengerowski
Three-quarters of all pharmaceutical drugs and supplements are administered as tablets and capsules based on active pharmaceutical ingredients (API). These are mostly powder mixes and cannot be processed directly in tabletting machines to produce pharmaceuticals. In order to convert the solids economically and gently into the end product, the powder is transformed into granulates of higher density. These can be dosed precisely, and during processing they cause considerably less dust accumulation in the working area than powders. This also reduces the health risks for staff to a minimum.
Powders to granulates
Chemgineering plant designers usually apply one or more of three methods to process active pharmaceutical ingredients. Compactors, fluid bed granulators or high shear mixers are selected for the granulation process. In compactors, the powder mix is fed from a supply or intermediate vessel to the compactor rolling press and processed under high pressure. The resulting pellets can be crushed to the desired particle size for further processing. The compactor technology offers the advantage that it is a purely physical process and the products are not chemically altered.
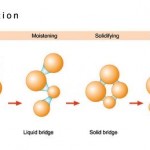
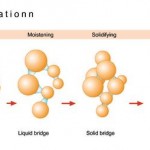
A fluid bed granulator delivers looser, blackberry-shaped agglomerates. The granulation takes place in a fluidised bed, and the drug mixture is generated and held in balance by an air flow from below. Spray coating the powder initiates agglomeration. With this method, both granulating and drying can be performed in one apparatus. However, during this procedure additional steps are required, like the prep- aration of the supply and exhaust air and cleaning of the filter elements in the exhaust stream.
The high shear mixer produces snowball-shaped, mechanically stable granulates. The apparatus consists of a cylindrical vessel with two stirring units and ancillary equipment. The active ingredient powder mix is prepared and, in the same vessel, spray coated with liquid in order to induce the granulation process. This process includes thorough mixing with the central stirring unit. Large granules and clumps can be broken up with the chopper affixed at the side. In the one-pot model of the high shear mixer, the granulate is also dried using vacuum, heatable vessel walls and microwaves.
After each of the described procedures, the dried granulate can be either added directly to the tablet press or first broken up to the desired particle size by a screen grinder. No matter how many choices there are, Chemgineering Technology designers always select and modify the methods to be used in accordance with the client’s internally defined procedures. GMP guidelines stipulate that the approval of a drug requires a minute description and guarantee of all manufacturing processes right down to the individual process steps. Even small alterations to the procedure may lead to changes in the active ingredient reaction and therefore necessitate amendments and re-releases by the regulatory authorities. Despite the many possibilities, the safest policy is always to rely on established operating procedures in the company.
Cleaning: WIP or CIP?
Cleaning the plant between single production steps serves to remove most of the drug residues and to protect people and products against contamination. In the context of highly active substances, manufacturers work mainly with closed purification processes: washing in place (WIP) or cleaning in place (CIP). Both cleaning methods use liquids applied to contaminated surfaces through cleaning nozzles in order to bind solid residues. The WIP method achieves a very high degree of cleaning, namely 90 to 95 %, especially in complex systems such as tablet presses. In most cases, this is sufficient to allow the facility to be opened without the operator’s health being threatened by the highly active substances. For fine cleansing, however, the plants need to be dismantled and cleaning completed manually. If the process needs to be fully validated and reproducible, it is referred to as CIP. In contrast to WIP, the manual cleaning step can be omitted here.
The technical effort involved in planning a WIP/CIP plant is by no means trivial and calls for various criteria to be considered. For example, the design of machinery and components needs to allow for WIP or CIP cleaning, in other words it should include many smooth surfaces and no dead spaces. Since the main cleaning action is achieved by the mechanical effect of the cleaning jet, the cleaning nozzles have to be set to the optimum water flow and pressure. If the water pressure and flow settings are too high or too low, the cleaning effect will be insufficient. Chemical detergents like tensides, acids or alkaline solutions can help here. Their cleaning intensity can be optimised by adjusting the temperature and the exposure time. The question of waste water treatment also needs to be taken into account at the planning stage. If dissolved substances must be pre-treated before being discharged into a waste water treatment plant, the process equipment concerned has to be coordinated. Furthermore, especially in the pharmaceutical sector, cleaning media and rinsing liquids are not usually recycled but used only once to prevent cross-contamination.
Typical projects
If solid forms processing needs to be added to an existing production line, each plant must be considered separately. In practice, older plants are not usually designed for WIP/CIP and retrofitting is very expensive. In most cases, it makes sense to aim for a new, closed processing line from the outset.
Chemgineering developed and installed a new production line for active ingredient processing on behalf of a blood pressure medication manufacturer, for example. A solid forms production line was already in place and the building in which the new facility was to be installed had already been fixed. A “closed“ process was designed, including double butterfly valves on the goods and materials transportation routes. Compared to single valves, the double type offers better protection against contamination. In line with the client’s approved procedures, a fluid bed granulator and high shear mixer with coater were selected for active ingredient processing.
The existing resources can also be used to handle small-scale projects. For a manufacturer of speciality pharmaceuticals, the Swiss facility planner designed a laboratory in which analytical samples were to be produced for research purposes. As many activities in this laboratory are performed manually, the systems had to be kept open. All three granulation methods were included in the design to facilitate flexible use of the lab. To enable mobile operation, the fluid bed generator and the high shear mixer were put on wheels as a way to simplify transport for cleaning or use. Wetting the product contacting surfaces is sufficient for cleaning. The engineers therefore designed a manual cleaning process with just water and waste water connections and a small number of cleaning nozzles.
Hall 9.1, Booth S37
Online-Info www.cpp-net.com/2209460
Share: