Enhanced flexibility and increased production volumes are constantly posing new challenges to the performance of the components used in biopharmaceutical production. Standardised units designed for quick scale-up support manufacturers in implementing their complicated processes. The crossflow filtration units in the Sartocon product family make true scalability a reality – under identical hydrodynamic conditions and with identical membranes.
Frank Meyeroltmanns
The individual steps of an integrative biopharmaceutical fluid handling process comprise media manufacturing, fermentation, cell harvesting, clarification, isolation and fine purification of the product, down-concentration of viruses, ultrafiltration all the way up to the final sterile filtration cycle and finally product filling. Several of the most important filtration steps involve ultrafiltration membranes and crossflow technology. Amongst other things, crossflow filtration is used in the following downstream process steps:
- Concentration to reduce volume
- Diafiltration to remove undesirable substances
- Changing buffer solutions
- Volume reduction for the final formulation
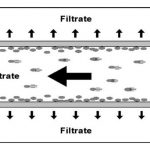
In crossflow filtration, the flow is directed tangentially across the ultrafiltration membrane. The driving force in the separation process is the transmembrane pressure (TMP). In accordance with the retention rating, undesirable substances are allowed to pass through the membrane, while the target proteins are retained. The feed rate at which the fluid flows constantly removes the laminar boundary layer in the flow channel and thereby prevents the formation of a cake layer. In order for this technology to be implementable in pharmaceutical processes, the crossflow filter must be manufactured with materials acceptable for pharmaceutical operations and also easy to clean. Simple handling can speed up the pace of the validation. Other requirements include a compact design, scalability and a low rate of product adsorption to prevent product loss. A high retention rating for the target agent is obviously a basic prerequisite when developing the process. It is therefore paramount that the right membrane is selected for the ultrafilter early in the biopharmaceutical development phase. Once this choice has been made, pilot tests are performed under dynamic conditions. The crossflow technology is tested for volumes of around 200 ml. Protein concentrations and process parameters can both be tested variably. Special attention must be paid to the TMP and the crossflow rate.
Quick scale-up
By using smaller-scale crossflow filters like Slice 200, valuable results can be achieved even under laboratory conditions and subsequently migrated to production scale. The Sartorius product families in the Sartocon series allow the necessary hydrodynamic comparability in that the same materials are used, the crossflow length is equivalent to the production filters and the process parameters (pressures, flow rate) are maintained. Process optimisations can thus also be simulated in the lab. Membrane screening, process parameter optimisation and cleaning optimisation are just a few of the many potential applications. After a membrane has been selected for a downstream process and its process parameters validated, the next step is to choose suitable, scale-compliant crossflow cassettes. One important aspect for the user is that these cassettes should be easy to install.
Monolithic
If large volumes are involved, larger filter surfaces are built into one block (Sartocube). Thanks to the integrated sealing technology, there is no longer any need for additional gaskets between the individual cassettes. In addition to its scalability, Sartocube offers further advantages: since five Sartocon cassettes have been replaced by a single block, it is not necessary to carry out five separate integrity tests. This significantly reduces the time and effort for testing and at the same time simplifies documentation and certification. All crossflow filter cassettes are installed in special filter holders. The process flows in the vertical direction in order to remove any trapped air at the beginning and achieve maximum product yields at the end through hydrostatic draining. The crossflow cassette design is cGMP-compliant, featuring validated compatibility with the majority of biopharmaceutical purification applications. The Sartocube filter unit has a minimum residual working volume and maximum flow rate along with enhanced mechanical and chemical stability. The cassettes themselves can be replaced quickly and easily thanks to the hydraulic holding device.
High-performance membranes
Ultrafiltration membranes made of polyethersulfone have become well-established in a wide variety of downstream processes. Polyethersulfone membranes are characterised by ruggedness and high performance. They allow conventional production processes to continue to be controlled efficiently in the future. Constantly changing demands like higher yields, increased production volumes and a growing number of licensed agents simultaneously necessitate new membranes for the ultrafiltration step. The monolithic Sartocube crossflow cassette combines easy handling with the high performance of Hydrosart membranes. The membrane is made of cellulose based material and exhibits high pH stability. Membrane regeneration, storage and depyrogenation are simple and can be carried out at high temperatures with NaOH. Thanks to the membrane material’s extremely hydrophilic properties, protein binding and fouling are reduced to virtually zero. These properties make Hydrosart the ideal solution for biological applications. The Sartocube series masters the tough challenges presented by the purification of valuable proteins and helps streamline the production of new and established medicines to achieve even greater efficiency.
cpp 457
Filtrations systems
Membrane Guide
Share: