Sanofi-Aventis produces approximately 5.2 billion tablets, 600 million capsules and 30 million ampoules of injectable solution each year at its Ambarès plant in France. Tracking production statuses and cost-effectively meeting regulatory requirements in the countries where the products are manufactured and sold is no small feat. The company therefore uses a control system with ControlLogix-based architecture.
To guarantee high-quality products, pharmaceutical manufacturers must keep up with rigorous and regularly modified compliance regulations for pharmaceutical testing and manufacturing. Sanofi-Aventis worked with Rockwell Automation to implement a control and visualisation solution at its Ambarès plant in France that would help it achieve FDA 21 CFR Part 11 compliance regulations and improve its tracking process. On one of its production lines, the Ambarès plant manufactures injectable liquid. After the liquid is created, the mixture is stirred and poured manually into tanks according to recipe specifications. The final solution is then stored in ampoules to preserve the product before it is shipped. The tanks are cleaned and prepared for the next batch after each operation. Faced with this disconnected and manually intensive process, Sanofi-Aventis‘ managers aimed to improve existing processing methods to reduce the risks associated with human error and increase efficiency. They began by investigating ways to optimise the aseptic process, focusing on Clean In Place (CIP) and Sterilise In Place (SIP), implementing new water-for-injection (WFI) loops for this purpose. WFI purifies water by distillation, rendering it sterile and preventing microbiological contamination.
Specifically, the company sought to achieve compliance with US and European pharmaceutical regulations for the next five years, mainly by tracking and controlling the WFI process according to 21 CFR Part 11 specifications. To meet its compliance goals, Sanofi-Aventis evaluated various control system platforms, including distributed control systems (DCS), programmable logic controllers (PLCs) and other automation solutions from vendors in the US, Europe and Japan. Company managers met with Rockwell Automation representatives and decided that a distributed, open control system would best meet their needs. “Representatives from Rockwell Automation demonstrated in-depth knowledge on more than just automation,” says Serge Landreau, Project Manager at Sanofi-Aventis. “They really understood the constraints associated with the pharmaceutical manufacturing process and were willing to work with us to find the right solution.”
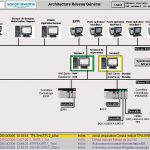
Centralised control platform
The collaborative venture between Sanofi-Aventis and Rockwell Automation began in February 2003. Based on a full analysis of the current manufacturing process, the team installed an integrated architecture with two Allen-Bradley ControlLogix controllers at its core. ControlLogix is a centralised control platform that combines sequential, multi-axis and process control in a single device, enabling it to be used as a central communications hub for the whole of the control architecture. As only one system is needed to control all functions, start-up and maintenance are significantly simplified and the costs for the development of new systems reduced. The RSView Supervisory Edition HMI software and PanelView HMI terminals also were incorporated. RSView SE is an HMI product for supervisory tasks and control applications. It supports decentralised server or multi-user applications, ensuring efficient machine control in real time. RSView SE was chosen for two main features: redundancy and controlled access to specific functions via the electronic signature feature in a Windows-dominated control environment. The redundancy function is divided between the HMI server and the data server. Sanofi-Aventis uses the redundant HMI server for continuous process monitoring. Even if the time for the individual processes does not last long, there are certain critical moments at which they are not allowed to be stopped. Even if the main HMI server fails, the operator can still monitor the process without any disruption.
Data loss prevented
It is also crucial for the company to trace data for regulation and quality purposes, which is why the RSView SE data server was set up in redundant mode to prevent any data loss. The overall system is secure for monitoring, regulation and quality issues. The switchover time to this application is less than one second. The electronic signature excludes some users from specific actions, such as modifying the settings of key parameters for quality, cleaning and sterilisation operations. Linked to the company domain controller, individual user names and passwords are supplied by the IT department. With the previous system, all 20 Sanofi-Aventis employees had access to the manufacturing process via the HMI system. With the current system, only four employees – out of the 20 with access to the process via the seven RSView SE clients – are allowed to access both the cleaning and sterilisations functions via the electronic signature and only two are authorised to control these actions. This ensures that only genuinely authorised personnel can make changes to recipes, thus improving the safety and security of the products. Thanks to the integrated control solution from Sanofi-Aventis, engineers can now confirm that all pipes and tanks are sterilized before a new process starts. Because the process is completely automated, they can also make sure that the right raw materials go into the right pipes and tanks, at the right time.
Improved process flow
In addition to the above process enhancements, the company simultaneously improved the productivity of its employees by eliminating non-valuable tasks traditionally performed manually, such as cleaning and sterilisation. This allowed highly qualified staff to concentrate on core aspects of corporate business. Rockwell also designed a training package for all operators and maintenance engineers using the system. The package included training in ControlLogix, networks and drives as well as customised training for users and maintenance specialists. The customised training sessions helped improve the skill levels of the maintenance team. The company additionally benefits through:
- Improved process quality and security. The system’s electronic double signatures help protect product integrity and streamline changes.
- Controlled costs and structured planning. Sanofi-Aventis has rationalised its planning activities and now allocates resources more cost-effectively.
- Traceability and real-time information. Total availability of monitoring and data throughout all manufacturing phases helps assure 21 CFR Part 11 compliance.
cpp 446
Programmable Logic Controllers
Automation University
Hannover Messe 2007
Sanofi-Aventis
Share: