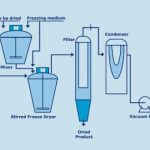
Stirred freeze drying is a quicker and less labour-intensive freeze drying process. It allows the production of loose and free-flowing powders at low temperatures and low pressures, all in one vessel. The main application of stirred freeze drying is in pharmaceutics, where 30 % of all antibiotics, 90 % of macromolecules and 50 % of electrolytes are produced in this way.
Dr. Peter G. J. van der Wel
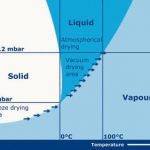
Discovered about one hundred years ago, freeze drying – also called lyophilisation – made a tremendous leap in the last century. From no more than a curious lab experiment, it has evolved into a mature production technique for materials which are instable at room temperature with their natural moisture content. Freeze drying involves the sublimation of a frozen solvent. After freezing, the substrate is placed in trays in a chamber, and by applying a deep vacuum the solvent is sublimated from the solid state directly into the vapour phase. The typical vacuum levels are between 1 and 0.01 mbar. When water is sublimated, for example, these deep vacuum levels ensure sublimation temperatures of -20 °C degrees or lower. Freeze drying results in a product with good shelf stability, which remains unchanged after reconstitution with the solvent. The freeze drying process freezes the material to be dried in its original structure and the solvent is removed from this structure without changing it.
One disadvantage of the conventional tray type of freeze dryer is the formation of lumps when the material is dried on a large scale. Despite the optimal structure of the individual product particles, the layer on the plates usually forms a single piece of hard-baked material. Another drawback can be the relatively low heat transfer rate due to the quiescent state of the material. The product often has to be crushed after freeze drying, sometimes leading to damage to the product structure. All these production process steps involve labour-intensive handling of material in trays, making this unique technology one of the most expensive unit operations in the pharmaceutical industry.
Advantages of the stirred freeze dryer
Early experiments with a conventional vacuum dryer operating at low temperatures and pressures showed that it is possible to use a freeze dryer under stirred conditions. The result is a lump-free, free-flowing product. Stirred freeze dryers also exhibit a better heat transfer rate due to the continuous mixing of the product, which shortens the drying process. Finally, the freezing phase is simplified because all steps can be carried out in a single processing unit instead of having to transport trays filled with product between freezing units, drying chambers and crushers. This makes for easy handling of the product, especially compared to traditional tray dryer equipment.
All in one vessel
The positive results achieved with these initial experiments have led to the development of a commercial stirred freeze drying technology by Hosokawa. The material to be dried is frozen in a stirred drying chamber with a dedicated design. The stirring motion causes the material – which can be liquid, pasty or solid – to be transformed into solid granules. The granule sizes and shapes are determined by the mixing characteristics of the machine. Once the freezing step is complete, the drying chamber is closed and vacuum is applied. Evacuation of the freezing agent is followed by the sublimation process. From this stage onwards, the product temperature is dictated by the vacuum level. During sublimation the heat is supplied through the jacket and efficiently distributed by the stirrer throughout the prod-uct. The initially coarse granules are gradually reduced in size due to the sublimation of the connecting ice structure between the frozen material. The released dried particles form a loose powder. Towards the end of the drying process, when most of the frozen solvent has been sublimated, the product temperature starts to rise. The product temperature is ultimately equal to the wall temperature, indicating that the drying process has finished. By then, all the material has been transformed into a fine, loose powder.
After breaking the vacuum, the dryer can be discharged easily from the dryer vessel, assisted by the transporting characteristics of the mixing element.
Applications
The main application of stirred freeze drying is in pharmaceutics, where 30 % of all antibiotics, 90 % of macromolecules and 50 % of electrolytes are produced in this way. Other products typically suited for freeze drying include proteins, hormones, viruses, vaccines, bacteria, yeasts, blood serum, liposomes and transplant materials like collagen sponge. Probiotic drying represents a particularly outstanding application for stirred freeze drying technology. The decisive arguments in favour of freeze drying with all these products are the preservation of the product structure, the particle size and the minimal temperature load.
Another fast-growing market for stirred freeze drying on a larger scale is the ma- terials sector, notably nano-materials. The use of this technology for the wet base processed materials implies a number of special advantages. The suspended particles remain separated during both the freezing and drying steps. Individual particles are separated in the course of sublimation, but the continuous mixing of the material induces the formation of weak agglomerates. The final product consists of loosely bound single particles forming a fine cohesive powder.
From lab scale to bulk quantities
The advent of stirred freeze drying has opened up new opportunities in production technology. The batch volumes of a stirred freeze dryer can range from a few litres for lab and small-scale applications to bulk drying of several cubic metres. Whatever the size, the advantages are obvious: rapid drying, simple product handling and a unique product quality.
Hall 5, Booth D38
cpp 459
Hosokawa Micron Group
Achema 2006
Share: