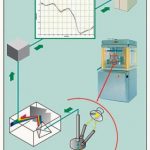
The use of near-infrared spectroscopy (NIR) for broader in-process control makes it possible to determine the active content of each tablet whilst it is still in the machine. The key benefits offered by the VisioNIR system are speed – it functions at the operating speeds of all currently used tableting machines – and real-time analysis of each individual product.
Inline methods of analysis are receiving increasing attention in the current discussion on process analytical technologies (PAT). Uhlmann VisioTec and Fette are currently investigating near-infrared (NIR) spectroscopy as a method for achieving 100 % control of tablets whilst they are still in the tableting machine. The results are extremely promising. Pharmaceutical regulations require accurate analyses of finished tablets as proof that the stated dose of active substance is actually present in the tablet. The pharmaceutical industry itself also has good grounds to monitor and control its production because this can help prevent the manufacture of costly reject batches and avoid costly product recalls that can seriously damage a corporation’s public image. Even when production is strictly in accordance with GMP and a company’s own standard operating procedures (SOP), transient problems resulting in defective products can occasionally occur.
Both the European Pharmacopoeia and the FDA only specify testing of small random samples of the finished product to verify the quality of batches of several hundred thousand tablets. The outcome is that existing, conventional procedures only enable analyses of active substance content on random samples of a product after the whole batch has been completed and in time-consuming tests that tie up significant amounts of lab capacity. Internal operating procedures introduced by the pharmaceutical industry for the purpose of enhancing drug safety call for an increased level of finished product sampling. Higher levels of in-process and finished product sampling help the pharmaceutical manufacturer identify trends in production. Very quick analysis of these samples is essential if the results are to be used to regulate operation of the production installations.
Pharmaceutical drug safety
Sampling procedures cannot detect all defective tablets, because each sample contains only a few specimens. The causes of out-of-specification (OOS) products can, for example, include transient production problems or the separation of pre-compaction mixtures. The outcome is that the dose contained in the product does not conform to specification. The number of product recalls appearing in pharmaceutical trade journals is clear evidence that production and marketing of undiscovered OOS batches is by no means a rare occurrence, even in Germany. A procedure for automatic quality control of all products which does not involve their destruction offers an opportunity to detect and segregate all those not conforming to specification. The quality of every single tablet produced can be tested and guaranteed, irrespective of batch size. The only way to ensure complete quality control of tablets while the machine is running is to use analytical methods that produce sufficiently quick results and do not destroy the products in the process. Spectroscopic methods meet both of these criteria.
In-process control
The VisioNIR system incorporates an NIR spectrometer and a special statistical evaluation program. The design and the measuring methods used by the spectrometer differ significantly from those of conventional models. The array of NIR photodiodes is specially designed to record spectra of rapidly moving samples. The Visio-NIR’s statistical evaluation program can process data and identify products at lightning speed, using comparisons of the spectra measured in the tablets with the input reference spectrum. The NIR reflection spectra of all tablets passing through the machine are checked during continuous production. In future, an ejector mechanism is planned that will automatically segregate and eject from the Fette tableting machine all tablets not conforming to the quality specification, without affecting the machine’s speed of operation. The NIR measurement taken by VisioNIR inside the machine uses the reflection procedure to determine the tablet’s exact active substance content immediately after the granules have been compressed.
In order to realise this procedure, it is necessary to develop a method of determining the active substance content with VisioNIR. Since this is a secondary method, feasibility tests first have to be performed and calibration curves recorded. Quantification of the active substance content by NIR is only possible if a validated reference analytical procedure exists. This requires a selection of independent specimen calibrations covering an adequate range and with physical properties corresponding to those of the production batches.
A scientific study has shown that compression force and, consequently, the tablets’ resistance to breakage have a strong influence on NIR spectra. Due allowance must be made for these factors when developing methods for NIR determination of the active substance content in solid pharmaceutical preparations. Excessive differences in resistance to breakage between the samples used for calibration and the actual production batches must be avoided. NIR determination of tablet content is only possible if the matrix of the tested tablets corresponds spectrally with that of the calibration samples, as otherwise the results could be falsified. In addition to chemical composition and resistance to breakage, other physical factors can influence the measurements obtained. These include temperature and pressure, variations in particle density, particle size and form, modification and water content, all of which can alter the spectra of one and the same sample. In order to facilitate comparisons of sample spectra with the reference spectrum, the result is displayed diagrammatically. The method must then by validated in accordance with recognized procedures before it can be successfully employed in production.
With the procedure currently used, the VisioNIR data is displayed on a separate PC. At some time in the near future, it is planned to transmit the data to the Fette NIR software, which will prepare complete production logs and intervene directly in the compression process if the programmed tolerances are exceeded.
Advantages
NIR measurement with a VisioNIR inside the tableting machine offers several important advantages:
- Non-destructive product analysis
- No time-consuming sample preparation
- Automatic quality control
- 100 % active substance control during production
- Rapid analysis (200 measurements per second)
The procedure offers many major benefits for the pharmaceutical industry by slashing the procedural delays before release of the finished product into circulation. It also greatly simplifies internal processes to yield lower process costs, higher process reliability, considerably lower lab and analysis costs and shorter release times as well as progress in know-how and competence.
Tablet press cpp 457
VisioNIR cpp 458
More informationen about the tableting machines
More informationen on VisioNIR
Achema 2006
Share: