The market for stick pack applications in the packaging of pharmaceutical products is growing steadily. The reasons for this rising popularity are obvious: minimal material consumption, a fast and safe packaging process and a convin-cing packaging system with a small footprint. This handy packaging style is also increasingly used in China. Bright Future, the supplier of pharmaceutical prod-ucts, is currently changing the packaging of penicillin from sachets to stick packs, for example.
The author: Frank Bühler Product Manager, Bosch Packaging Systems
Established in 1993, Bright Future Pharmaceutical Ltd. has grown into Hong Kong’s largest GMP (Good Manufacturing Practice) certified supplier of pharmaceutical products. In the meantime, the company operates six fully equipped production plants and commits itself to improving people’s health by making high-quality pharmaceuticals using state-of-the-art technology. Until now, Bright Future has marketed penicillin powder packaged in sachets (flat pouches). It was an increase in sales that lay behind the recent decision to search for a packaging solution that consumes less material. Stick packs proved to be a suitable option. Bosch Packaging Systems was able to offer a packaging line fulfilling all the required parameters for both performance and quality. Apart from high demands regarding pouch integrity and powder dosing accuracy, it was considered crucial to ensure lean processes throughout the project lifecycle as a way to minimise resource usage. The stick pack itself has a perforation to permit efficient and safe opening, easy tearing and fast and complete emptying of the package. Bright Future now markets its penicillin as a flow-wrapped multipack containing six single stick packs.
Complete system from one source
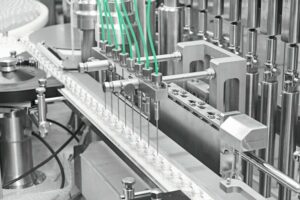
The requirements for a stick pack packaging line are highly complex. In addition to dosing accuracy, permanently high tightness of the primary packaging and safe interface handling are impor-tant factors. Dependable secondary packaging is equally essential. The Bosch PLC system combines maximum reliability with a small footprint. Both the primary and secondary packaging are offered by a single supplier. Primary packaging into stick packs involves precise dosing on the ten-lane vertical Sigpack RA machine. The subsequent secondary packaging process is carried out on the horizontal Sigpack HCM flow wrapper. With an output of 175 packages, the HCM flow wraps up to 1000 stick packs per minute.
Filling augers which are individually controlled by an AC servo motor guarantee the required dosing accuracy for the primary packaging operation. As the density of penicillin powder can be subject to fluctuations, every single stick pack has to be separately weighed. After the weight has been checked by an in-line weighing system, dosing at the machine is regulated by means of an integrated tendency control. This continuous adjustment ensures a consistently accurate filling process. Following the weighing procedure, the stick packs are counted, grouped and fed to the HCM flow wrapper. Each sales unit packaged on the HCM consists of two stacks of three penicillin stick packs each in a particularly flat and compact flow wrap. The secondary packaging is produced efficiently using less material and benefits the end user with easy handling.
GMP-compliant design
Ever since its foundation, Bright Future has been committed to the promotion of public health. The company attaches the very highest priority to the quality of its pharmaceutical products. Bright Future’s manufacturing processes adhere strictly to GMP and GLP (Good Laboratory Practice) principles. The line’s GMP-compliant design therefore meets a further customer demand. The cantilever structure means the process and drive units are completely separate from one another. Moreover, the ergonomic height improves operator access and allows a direct view into the packaging process. Format changes and the supply of packaging material can be realised quickly and easily. Lifetime-lubricated components also contribute to the line’s hygienic design.
The machine casing provides safety and noise protection. Underneath the product transport unit, the machine elements are fully visible to facilitate line clearance. Depending on the customer’s requirements, rejected products and waste can be collected either in special lockable containers or on catch plates.
Crucial operating functions such as start, stop and emergency stop are integrated on the front of the line. All other functions are accessible via a swivel-type human machine interface (HMI) with a 12” colour touch screen. The HMI is available with two different specifications for pharmaceutical applications. In addition to the basic version used by Bright Future, a second version achieves full compliance with the requirements of 21 CFR Part 11. It features integrated functions such as audit trail, electronic record and RAID data protection to ensure GxP-compliant operator and data management. A connection to a higher-level MES system is also possible.
The line enables Bright Future to package its penicillin powder using less material while increasing efficiency. Simultaneously, the easy-to-open stick packs enhance the convenience for end users. “To allow us to supply our global customer base with reliable pharmaceutical and health products, we were seeking a competent partner offering high-quality equipment. We found this partner in Bosch Packaging Systems”, says Dr Susanna Wan, Purchasing and Development Manager at Bright Future. She is looking forward to a successful conclusion of the project and the commissioning of the new line scheduled for summer 2012.
cpp-net.com/0113470
Share: