Today’s chemicals industry is subject to growing pressure; plants are required to perform a variety of chemical operations with varying process media at ever-shorter process intervals and in batch sizes ranging from small to large. As a result, the diverse range of applications offered by the materials used to construct these installations are presenting increasingly challenging requirements – those can be effectively fulfilled with the Hastelloy Hybrid-BC1 alloy from Haynes International.
Hybrid alloy offers a solution for plant manufacturers
All-purpose weapon in the battle against corrosion
Today’s chemicals industry is subject to growing pressure; plants are required to perform a variety of chemical operations with varying process media at ever-shorter process intervals and in batch sizes ranging from small to large. As a result, the diverse range of applications offered by the materials used to construct these installations are presenting increasingly challenging requirements – those can be effectively fulfilled with the Hastelloy Hybrid-BC1 alloy from Haynes International.
Corrosion damage is a serious economic factor; destructive chemical processes are a threat to steel structures such as bridges or girders and represent a costly problem for industrial installations, power plants and pipelines – to name but a few. The World Corrosion Organisation (WCO) estimates the economic damage caused by corrosion to amount to2.2 trillion US dollars per year.
Of course there are materials that are resistant to the advance of corrosion and are thus elevated to the status of “corrosion-resistant”. Classic stainless steels, for example, offer a useful basic level of corrosion protection – but soon reach their limits when faced with aggressive substances like inorganic acids or chloride environments. The limits of endurance of these steels are demonstrated by their susceptibility to stress corrosion cracking, corrosion pitting and crevice corrosion. Nickel puts up a better defence; pure nickel is the material of choice for applications involving process fluids with high levels of sodium and potassium hydroxide (caustic chemicals).
Zirconium and titanium alloys afford roughly the same level of protection as corrosion-resistant nickel-based alloys; however, as these reactive alloys are used in a variety of highly specific areas and are intolerant to specific ionic substances, they will not be examined further in this article.
Nickel-based materials thus remain as the alternative with the broadest effective range, resisting most forms of corrosion. They are extremely versatile and resistant to both oxidising and reducing acids and alkalis, and they also minimise the problems outlined above such as stress corrosion cracking, corrosion pitting and crevice corrosion. Nickel-based alloys are moreover extremely ductile, weldable and formable and the production of industrial components and fabrications is a relatively straightforward process.
One alloy for all media?
Sulphuric acid, for example, is a highly aggressive fluid that can occur in both reducing and oxidising forms. This is where nickel alloys such as the Hastelloy B and C types from Haynes International come in. Based in Kokomo, Indiana, Haynes International is a global leading developer and manufacturer of nickel and cobalt alloys and has been a partner of the Zapp Group for over sixty years. B type alloys comprise nickel-molybdenum alloys while C-type alloys include nickel-chromium-molybdenum alloys.
However, the corrosion process is determined not only by the process fluids themselves; contamination of these fluids represents a further factor that is often difficult or impossible to influence or predict. In practice, consideration of this factor to minimise plant or system corrosion would require different materials to be used for one and the same acid with different degrees of contamination.
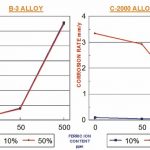
Tests involving boiling 10 % and 50 % sulphuric acid show the extent to which the two alloy types B and C are suitable for individual applications with pure and contaminated sulphuric acid respectively. Hastelloy B-3 alloy with approx. 30 % molybdenum and approx. 1.5% chromium shows outstanding corrosion resistance to pure reducing sulphuric acid in the two given concentrations, with negligible corrosion rates. However, as soon as even trace amounts of ferric ions are added to the acid, its corrosion resistance decreases – dramatically in some cases. The reverse can be observed in the curve for 50 % sulphuric acid, where the corrosion resistance of Hastelloy C-2000 alloy with 23 % chromium and 16 % molybdenum increases in parallel to a lower increase in the proportion of ferric ions. Ferric ions can often be found in process streams as a contaminant due to the corrosion of iron-containing alloys further upstream (Figure 2).
The problem is a classic case of “either / or”. In practice, the proportion of oxidising contaminants is frequently variable and the nature of the acid may change from reducing to oxidising or vice versa. Plant operators face the same problem when process fluids with opposing properties must be processed in rapid succession. New processes and product characteristics present a continuous stream of new challenges to material quality in plant engineering. To ensure that the field of plant operation both in and beyond the chemical industry retains its value and cost-effectiveness, a material is needed that can outperform classic B and C alloys by coping easily with these changes in fluid properties while also minimising corrosion. In fact, a material that is significantly more capable of handling both sets of requirements already exists; this material combining the best of both worlds is a hybrid alloy.
Hybrid solution
In technology, “hybrid” – a term derived from the Greek for “mixed, of two origins” – is generally used to denote a system combining two technologies. The prefix “hybrid” indicates a whole that is composed of differing types or processes, with the special feature that each of the combined elements represents an individual solution in itself – just as the materials listed in the example do for specific groups of process fluids – but, when combined, can create new and desirable properties: here, this would be the ability to process both reducing and oxidising materials at high temperatures in one and the same installation.
The new hybrid material – a nickel-based alloy, for the reasons outlined above – is also manufactured by Haynes. The Hastelloy Hybrid-BC1 alloy was developed to fill precisely the gap described, for which no truly effective solution had yet been discovered.
Best of both worlds
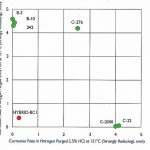
A further example of the ability of a single process fluid to change properties can be seen from a series of tests using hydrochloric acid. In Figure 3 the X axis shows the corrosion rate in mm / year for a strongly reducing 2.5 % solution of hydrochloric acid (HCl) at +121°C. The Y axis shows the same HCl with the same parameters, but with one difference: purging with oxygen instead of nitrogen causes the acid to have a strongly oxidising effect on the metal. In this case, the Hastelloy Hybrid-BC1 alloy (shown in the figure as a red dot) offers an excellent compromise by combining the strengths of the alloys in the B and C families, thus significantly reducing the failure risk of both these classic material types in many cases.
www.cpp-net.com search: cpp0315zapp
Reinhart Baden
Reinhart Baden
Technical Customer Service,Specialty Materials,Zapp Materials Engineering
Share: