Metal dusting is an extreme form of high-temperature corrosion which may occur in process environments at elevated temperatures with high carbon activity. In our interview with alloy specialist Reinhart Baden of Zapp Materials Engineering, we find out how metal dusting works, when it occurs and how it can be prevented.
High resistance to metal dusting
A magic bullet
Metal dusting is an extreme form of high-temperature corrosion which may occur in process environments at elevated temperatures with high carbon activity. In our interview with alloy specialist Reinhart Baden of Zapp Materials Engineering, we find out how metal dusting works, when it occurs and how it can be prevented.
Mr. Baden, can you give us an outline of what metal dusting involves?
Baden: Metal dusting is the term given to an extreme form of corrosion which occurs in process environments with high carbon activity. The presence of carbon leads to internal carburisation, which generally results in coke deposition. This causes the material to decompose into graphite and metal/alloy; the structure of the material disintegrates, producing visible pitting or brittleness.
What are the conditions where metal dusting occurs?
Baden: Metal dusting, the inevitable disintegration of metal alloys, primarily occurs at high pressures and temperatures between 550 and 850 °C, in carbonaceous atmospheres of H2, CO, CO2 and steam/gas mixtures.
What are the effects of metal dusting?
Baden: The process occurs in highly reductive and carburising atmospheres with extremely high carbon activity (ac 1) and is caused by the key reactions of CO + H2 to C + H2O and 2 CO to C + CO2. As a result, the metal components disintegrate into a “dust” of graphite and metal or metal carbide particles. Corrosion by metal dusting is thus an extremely severe problem.
What types of industrial plants and systems are susceptible to metal dusting?
Baden: Industrial processes and systems that are susceptible to metal dusting include methane reforming for the production and processing of syngas (CO + H2), industrial furnaces and systems for producing ammonia, methanol, dimethyl ether or hydrogen synthesis and GTL (gas-to-liquid) systems. Retorts, sleeves, baskets and piping are typical parts of petrochemical or energy generating plants that may be affected.
But isn’t metal dusting under control as far as the materials used are concerned? There are various materials containing chromium, aluminium or silicon alloys that can prevent it. So what’s the problem?
Baden: The problem is that corrosion resistance is not the be-all and end-all for a material! It’s enormously important for alloys to have other properties as well as high resistance to metal dusting. Good fabrication characteristics are an example. Cold working and welding are among the most frequently used production processes, particularly when manufacturing components for the types of systems I mentioned earlier. And this is where the problems start, when the downside of alloy components such as silicon and aluminium emerges. For example, a high aluminium content may cause stress cracking in an alloy during heat treatment after welding or forming. Silicon, on the other hand, will increase the material’s susceptibility to weld solidification cracking. The presence of aluminium and silicon, which are so desirable as protection against metal dusting, can thus lead to problems in forming, welding and other forms of processing.
In addition to good fabrication characteristics, thermal stability is also an important consideration for many high-temperature nickel alloys; the alloy must retain its desirable properties virtually unchanged even when in use at high ambient temperatures.
So is there a solution for this problem, too?
Baden: Yes, as a matter of fact there is. An ideal alloy combines resistance to metal dusting with properties such as outstanding fabrication characteristics and thermal stability. Bearing all these challenging requirements in mind, the research division at Haynes International, Inc. developed a new nickel-based alloy called Haynes HR-235. This material is composed of 31 % chromium by weight, 5.6 % molybdenum, 3.8 % copper plus several other minor elements and is marketed by Zapp Materials Engineering.
What properties does the material have?
Baden: Haynes HR-235 alloy is resistant to metal dusting and also offers high levels of resistance to general corrosion. It’s exceptionally easy to work, which – as I already noted – can by no means be taken for granted. As well as its excellent fabrication characteristics, especially in production processes such as cold working and welding, Haynes HR-235 alloy offers high thermal stability and retains its positive properties even at elevated temperatures. All these welcome features are due to the alloy’s high chromium content in addition to copper and various minor elements such as manganese and silicon. If the protective surface layer of chromium oxide is damaged, the copper below it slows the catalytic reaction. Given that iron exacerbates the metal dusting reaction, a further benefit of Haynes HR-235 alloy is its relatively low iron content of under 1.5 %.
What purposes is the alloy designed for?
Baden: The alloy can also be used with corrosive media such as sulphuric acid as well as alkaline media like caustic soda.
So you can say that HR-235 alloy is resistant to both high temperatures and corrosion?
Baden: Yes, the alloy can also be used for high-temperature and corrosive media provided it is adequately resistant to the aqueous media to which it is exposed.
What types of tests did you conduct on the alloy?
Baden: The metal dusting reaction is basically a catalytic process which is relatively slow but occurs in many nickel-based alloys after several thousand hours of exposure. Such a slow process is unsuited for rapidly obtaining robust test results to demonstrate the superiority of Haynes HR-235 alloy over other existing nickel-based alloys. The testing methodology selected for the study was therefore extremely aggressive in comparison with real-life industrial environments. Severe thermal cycling may develop cracking in the oxide scale, thus allowing the metal dusting reaction to initiate at an early stage. Minimising the incubation stage in this way significantly shortened the test period. Metal dusting tests are often laborious and expensive procedures, as realistic process conditions can rarely be simulated in the laboratory.
What were the results of the tests?
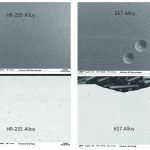
Baden: The tests run on the new alloy were designed to examine its resistance to metal dusting within a defined environment in comparison with another alloy. To achieve this, a microchannel testing device was built with one side made of HR-235 alloy and the other of 617 alloy. The microchannel formed a common flow channel for the steam methane reforming reaction and exposed both alloys to comparable metal dusting conditions. The microchannel device was tested at high pressure in a syngas environment for a total duration of approximately 1200 hours.
After completing the test period, the surface of the 617 alloy control material showed severe pitting; however, the HR-235 exhibited no signs of pitting after exposure to the same syngas environment. Surface and cross-sectional SEM analyses clearly confirmed this pitting effect in the 617 alloy.
What about the much-feared effect of carburisation in metal dusting environments? How susceptible is the new alloy to coke deposition?
Baden: In an environment favourable to metal dusting the elements affected often show weight change, which is generally due to oxidation, scale spallation, internal carburisation and – most important – coke deposition resulting from catalytic surface reactions. For this reason, coke deposition, pitting and internal attack are the primary effects investigated in test runs to demonstrate the resistance of HR-235 in metal dusting environments. As the operating temperatures at which metal dusting reactions tend to occur are relatively low, the effects of internal attack can be assumed to be not too severe; consequently, metal disintegration on the surface is likely to be the root cause of catastrophic failure due to metal dusting.
The coke deposition tests also delivered unequivocal results. They revealed a thick coke layer deposited on the Haynes 230 sample, while only localised coke formation was observed on HR-235. This was corroborated by an XRD analysis, which clearly indicated the presence of graphite in the 230 alloy sample but undetectable levels of graphite in the HR-235.
Overall, it was plain that HR-235 slowed the metal dusting reaction significantly but – unlike 230 alloy – without ever displaying the major disintegration that is inevitable in catastrophic mode.
So is HR 235 the ‘magic bullet’ of metals for use under metal dusting conditions?
Baden: Basically, yes! Let’s take the quality criterion of weldability, which I mentioned previously as so important for materials. The chemical composition of Haynes HR-235 alloy was designed for good weldability and Haynes has likewise delivered proof of its new alloy’s outstanding properties. Extensive welding tests (WIG, MIG) on 3 mm samples of sheet metal and 12.7 mm sheets of HR-235 alloy proved beyond doubt that HR-235 is as suitable for homogeneous and heterogeneous welding as the well-known Hastelloy C alloy family – but shows significantly higher resistance to metal dusting and can be used in a range of ambient conditions from room to high temperatures. Taken in conjunction with the outstanding properties of HR-235 alloy, the material really is a kind of ‘magic bullet’ among nickel-based alloys.
www.cpp-net.com search: cpp0216zapp
The problem is that corrosion resistance is not the be-all and end-all for a material!
Dr. Bernd Rademacher
Dr. Bernd Rademacher
Editor,
cpp chemical plants & processes
Share: