Sustained-release formulations that gradually release active pharmaceutical ingredients (APIs) have numerous therapeutic benefits, but they also involve time-consuming quality control. Near-infrared spectroscopy (NIRS) offers a fast and simple alternative to tedious dissolution testing: by using NIRS and appropriate calibration models, the release of APIs from sustained-release tablets can be predicted accurately within a matter of minutes.
Author Stephanie Kappes Technical writer, Metrohm
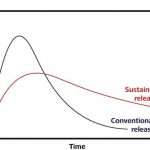
After taking a tablet, a phenomenon known as a plasma peak usually occurs: this is when the full amount of the drug is rap-idly released from the tablet and the active ingredient concentration temporarily reaches a high level in the patient’s blood plasma as a result. Depending on the type of drug, this high concentration can cause significant side-effects. In addition, a high administration frequency is required to produce a lasting effect, as the high plasma level that patients experience at the outset then falls rapidly again as the active ingredient is metabolised.
Sustained-release formulations, which release active ingredients gradually, offer a solution to these issues (Figure 3). The occurrence of a plasma peak is prevented and a reasonable plasma level is maintained over a longer period. In particular, hormone preparations, drugs for regulating blood pressure, painkillers and antidepressants are often administered in the form of sustained-release tablets.
There are various options available for creating sustained-release tablets. One approach involves applying a coating to the tablets to prevent them from decomposing rapidly in the gastric juice. In many cases, tablets also contain special polymers. These create a matrix structure that releases the API at a slow pace. In such tablets, the duration over which the entire active ingredient quantity is released can be manipulated through the polymer concentration in the tablet as well as the concentration of plasticisers, if these are used. Some examples of polymers that are commonly used in sustained-release formulations are ethyl cellulose (in combination with the plasticiser acetyl tributyl citrate, or ATBC for short) and Eudragit NE 30 D.
One drawback of sustained-release tablets is that they involve time-consuming quality control processes. In most cases, drug release is assessed by dissolution testing. To do this, the tablet is placed in a solvent that has similar properties to gastric juice. The free active ingredient is then determined at regular intervals over the desired release period – often 24 hours.
NIRS offers fast quality control
Near-infrared spectroscopy, however, offers an alternative to dissolution testing (Figures 1 and 2). By using NIRS together with an appropriate calibration model, it is possible to predict the dissolution behaviour of tablets accurately and within a matter of minutes. What’s more, this analysis method – unlike dissolution testing – is non-destructive, which allows larger sample quantities to be analysed. In a 2009 publication, Tabasi et al. describe the process of predicting the dissolution behaviour of theophylline tablets using NIRS (Int. J. Pharm. 382, 1–6). Theophylline tablets in a sustained-release format are used to treat asthma. The tablets that were investigated by Tabasi and her colleagues contained different concentrations of the polymer Eudragit NE 30 D, which slows the release of the active ingredient.
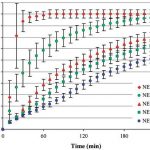
After recording the NIR spectra of several tablets with different polymer contents (0, 5, 10, 15 and 20 % Eudragit NE 30 D), the researchers determined the active ingredient release using a dissolution test (Figure 4). They used the data obtained from this to create a calibration model for each of the four measuring times (1, 2, 3 and 4 hours). These models correlated the polymer content-related changes in the NIR spectrum with the measured values from the dissolution tests.
Multivariate analysis is the key
When an NIR spectrum is recorded using a conventional spectrometer, the sample is irradiated using light with a succession of different wavelengths. In the process, a defined wavelength range is scanned in the near-infrared region. Depending on the sample properties, either the reflectance or the transmittance is measured. The spectrum is obtained by plotting this value against the wavelength. Representing a series of spectra in the form of a matrix enables multivariate analysis. Where there are n spectra from different samples, consisting of p measured values in each case (with p different wavelengths), the matrix has the following format:
In the case of the theophylline tablets, each row represents a certain polymer concentration and contains the reflectance values per wavelength, averaged over several samples and measurements. To create a calibration model with the commonly used partial least squares (PLS) regression, the components of the data matrix are projected onto a new, orthogonal coordinate system. The latter is chosen such that the data can be represented using as few components as possible. This method resembles principal component analysis (PCA).
Unlike PCA, however, PLS considers not only the NIRS data when defining a new coordinate system. Instead, the values obtained from the dissolution test are also taken into account in order to maximise the covariance and, therefore, the correlation of the NIRS data with the data from the dissolution test. This produces a model that is capable of predicting dissolution behaviour on the basis of an NIR spectrum.
Following validation, the models can then be used to predict dissolution behaviour in similar samples. Figure 5 shows a comparison of the predictions and measurements carried out by Tabasi et al. for a few selected samples from their validation set.
NIRS saves time and effort
Near-infrared spectroscopy offers a quick and simple way of predicting the dissolution behaviour of tablets. Provided a suitable calibration model is available for the type of tablet in question, the method is able to create a prediction within a matter of minutes. Of course, the prediction can only be as good as the model on which it is based, but a model that has been sufficiently validated will deliver reliable, accurate results.
When it comes to tablet quality control, this method reduces significantly the amount of time and work spent on the process. In addition, its speed, simplicity and non-destructive analysis make it possible to investigate significantly larger sample quantities than would be the case with dissolution testing.
cpp-net.com/0314402
Share: